Publication
Advanced Redox Technology Lab
Publication
Advanced Redox Technology Lab
Journal papers
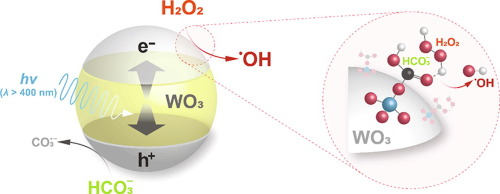
The generation of hydroxyl radical (•OH) by visible light-illuminated tungsten oxide (WO3) was found to be significantly improved in the presence of hydrogen peroxide (H2O2) and bicarbonate ion (HCO3−). A ternary system of hν/WO3/H2O2/HCO3− showed synergistic enhancement in oxidation of benzoic acid (BA, a •OH probe compound) into hydroxybenzoic acids (HBAs), exhibiting even less consumption of H2O2 than hν/WO3/H2O2. Analyses of HBAs from BA oxidation (three HBA isomers and 18O-labelled HBA from H218O2) suggested that hν/WO3/H2O2/HCO3−, contrary to hν/WO3/H2O2, generated •OH mainly via one-electron transfer from the conduction band of WO3 to H2O2. The dominant one-electron reduction of H2O2 over HCO3−-treated WO3 was further evidenced by Koutecký–Levich plots obtained with a rotating disk electrode setup. Based on different experiments using electron paramagnetic resonance spectroscopy, radical scavengers and probes, (photo-)electrochemical measurements, and density functional theory calculations, the mechanisms underlying the enhanced generation of •OH by hν/WO3/H2O2/HCO3− were discussed.