Publication
Advanced Redox Technology Lab
Publication
Advanced Redox Technology Lab
Journal papers
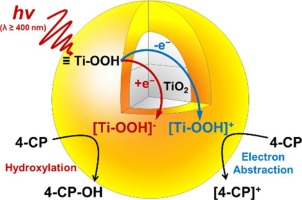
Amorphous peroxo-titania (denoted as Am-peroxo-TiO2), synthesized in this study by a facile method, demonstrated photochemical activity for the oxidation of organic pollutants under visible light illumination (λ > 400 nm). Am-peroxo-TiO2 was synthesized by a one-step sol-gel method using titanium isopropoxide and hydrogen peroxide (H2O2) at room temperature and atmospheric pressure. The material produced was a yellow powdered precipitate; the measurement of diffuse reflectance confirmed light absorption of up to 600 nm. High-resolution transmission electron microscopy revealed that Am-peroxo-TiO2 forms aggregates of small nanoparticles (ca < 10 nm). The surface peroxo-groups (Ti-OOH or Ti-OO-Ti) were characterized by Fourier-transform infrared spectroscopy and X-ray photoelectron spectroscopy. Visible light-illuminated Am-peroxo-TiO2 completely degraded 10 μM 4-chlorophenol (4–CP) in 4 h. The photochemical activity of Am-peroxo-TiO2 was selective to the target organic compound. Experiments using scavengers and probes of reactive oxidants revealed that reactive oxygen species such as hydroxyl and superoxide radicals are not responsible for the degradation of organic compounds. Liquid chromatography-mass spectrometry showed that 4–CP was oxidized to produce 4-chlorocatechol, hydroquinone, and benzoquinone as primary products. The results suggest that oxidation is initiated by electron abstraction or hydroxylation by the photogenerated reactive intermediates on the peroxo surface. Am-peroxo-TiO2 was stable under both dark and illuminated conditions in the absence of organic compounds. Importantly, in the presence of organic compounds, the photochemical activity of Am-peroxo-TiO2 gradually decreased. Further, platinization enhanced the photochemical activity as well as the stability of Am-peroxo-TiO2.