Publication
Advanced Redox Technology Lab
Publication
Advanced Redox Technology Lab
Journal papers
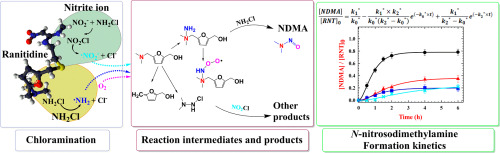
Ranitidine (RNT) has been an important tertiary amine precursor of N-nitrosodimethylamine (NDMA) in chlorine-based water treatment, due to reaction with monochloramine (NH2Cl) with exceptionally high molar yields up to 90%. This study examined the effects of nitrite ions (NO2−) on the kinetics of NDMA formation during the chloramination of RNT under variable concentrations of dissolved oxygen (DO, 0.7–7.5 mg/L), RNT (5–30 μM), NH2Cl (5–20 mM), NO2− or NO3− (0–2 mM) and pH (5.6–8.6). In the absence of the NO2−, the ultimate molar yield of NDMA after 6 h of reaction was primarily influenced by [DO] and pH, while marginally affected by initial [RNT] and [NH2Cl]. A kinetic model, prepared in accordance with the reaction sequence of NDMA formation, suggested that the rate determining step was accelerated with increasing [NH2Cl]0, [DO], and pH. A Kinetic study together with ultra-performance liquid chromatography-quadrupole-time of flight mass spectrometer (UPLC-Q-TOF MS) and gas chromatography (GC)/TOF MS analyses in parallel demonstrated that the nitrite ion inhibited the nucleophilic substitution of the terminal amine on NH2Cl, and reduced the pseudo-steady state concentration of N-peroxyl radicals, significantly decreasing the ultimate yields of NDMA.