Publication
Advanced Redox Technology Lab
Publication
Advanced Redox Technology Lab
Journal papers
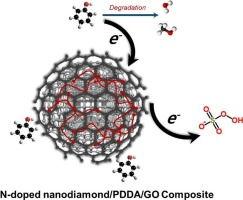
Changes in surface carbon hybridization through high-temperature annealing (>1000 °C) of nanodiamond (ND), i.e., surface graphitization, enable peroxymonosulfate (PMS) activation by ND. Alternatively, this study suggests low-temperature surface modification (500 °C) of ND as an effective strategy for allowing ND to activate PMS. ND calcination in the presence of poly(diallydimethylammonium chloride) (PDDA) and graphene oxide (GO) in an NH3 atmosphere produced binary and ternary nitrogen-doped ND composites (i.e., N-ND/PDDA and N-ND/PDDA/GO). Compared with bare ND, theses surface-modified NDs markedly enhanced organic oxidation associated with PMS activation. In particular, N-ND/PDDA/GO outperformed graphitized ND in terms of PMS activation capacity. Spectroscopic characterization implied that the content of pyridinic N and the N doping level increased with further modification of ND. Oxidation by PMS activated with ND-based materials did not involve radical attack, as methanol did not exhibit a quenching effect, formaldehyde yield was insignificant, conversion of bromide into bromate was negligible, the substrate specificity contradicted sulfate radical (SO4radical dot−) reactivity, and no electron paramagnetic resonance spectral features were assignable to SO4radical dot− adducts. Impedance spectroscopic analysis indicated a high correlation between PMS activation efficacy and electrical conductivity. Chronoamperometric measurements showed that sequential injection of PMS and 4-chlorophenol caused current generation at electrodes coated with ND-based activators, and the increase in current intensity correlated well with PMS activation capacity. These findings suggest that ND-derived materials facilitated the electron transfer from organics to PMS, resulting in a degradative reaction route not reliant on radical species.