Publication
Advanced Redox Technology Lab
Publication
Advanced Redox Technology Lab
Journal papers
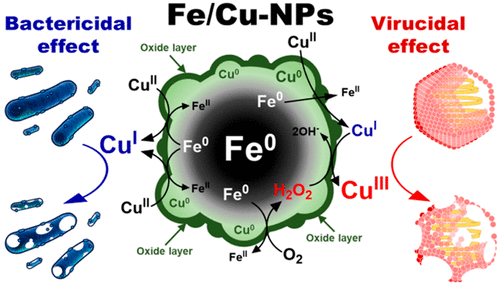
Bimetallic iron–copper nanoparticles (Fe/Cu-NPs) were synthesized by a single-pot surfactant-free method in aqueous solution [via the reduction of ferrous ion to zerovalent iron nanoparticles (Fe-NPs) and the subsequent copper-coating by metal ion exchange]. The produced Fe/Cu-NPs formed aggregates of spherical nanoparticles (approximately 30–70 nm) of Fe–Cu core–shell structures with 11 wt % copper content. The microbicidal effects of Fe/Cu-NPs were explored on Escherichia coli and MS2 coliphage, surrogates for bacterial and viral pathogens, respectively. Fe/Cu-NPs exhibited synergistically enhanced activity for the inactivation of E. coli and MS2, compared to single-metal nanoparticles (i.e., Fe-NPs and Cu-NPs). Various experiments (microbial inactivation tests under different conditions, fluorescence staining assays, experiments using ELISA and qRT-PCR, etc.) suggested that Fe/Cu-NPs inactivate E. coli and MS2 via dual microbicidal mechanisms. Two biocidal copper species [Cu(I) and Cu(III)] can be generated by different redox reactions of Fe/Cu-NPs. It is suggested that E. coli is strongly influenced by the cytotoxicity of Cu(I), while MS2 is inactivated mainly due to the oxidative damages of protein capsid and RNA by Cu(III).