Publication
Advanced Redox Technology Lab
Publication
Advanced Redox Technology Lab
Journal papers
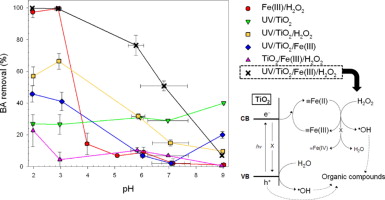
The integration of two different AOPs often offers synergistic reaction routes for the production of  OH. In this study, synergistic production of
OH. In this study, synergistic production of  OH was observed in the combined system of TiO2 photocatalysis and the Fenton-like reaction, causing a drastic enhancement in the oxidation of organic compounds at circumneutral pH values. The photolytic experiments using organic substrates (i.e., phenol, benzoic acid, and methanol) and valence band hole and
OH was observed in the combined system of TiO2 photocatalysis and the Fenton-like reaction, causing a drastic enhancement in the oxidation of organic compounds at circumneutral pH values. The photolytic experiments using organic substrates (i.e., phenol, benzoic acid, and methanol) and valence band hole and  OH scavengers (i.e., formate and tert-butyl alcohol) show that the synergistic effects result from dual roles of iron as an electron acceptor to facilitate charge separation in TiO2 photocatalyst and as a Fenton reagent to catalyze conversion of H2O2 into
OH scavengers (i.e., formate and tert-butyl alcohol) show that the synergistic effects result from dual roles of iron as an electron acceptor to facilitate charge separation in TiO2 photocatalyst and as a Fenton reagent to catalyze conversion of H2O2 into  OH. A noteworthy observation is that the adsorption of iron onto the photoexcited TiO2 surface possibly modifies electron transfer properties of iron toward H2O2 at neutral pH to convert the resultant reactive oxidant from Fe(IV) into a stronger form, likely
OH. A noteworthy observation is that the adsorption of iron onto the photoexcited TiO2 surface possibly modifies electron transfer properties of iron toward H2O2 at neutral pH to convert the resultant reactive oxidant from Fe(IV) into a stronger form, likely  OH.
OH.