Publication
Advanced Redox Technology Lab
Publication
Advanced Redox Technology Lab
Journal papers
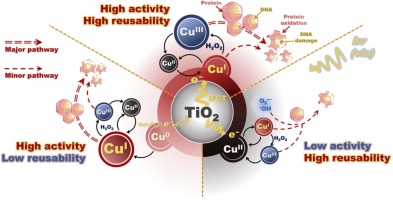
Copper (Cu) is an effective antimicrobial material; however, its activity is inhibited by oxidation. Titanium dioxide (TiO2) photocatalysis prevents Cu oxidation and improves its antimicrobial activity and stability. In this study, the virucidal efficacy of Cu-doped TiO2 nanoparticles (Cu-TiO2) with three different oxidation states of the Cu dopant (i.e., zero-valent Cu (Cu0), cuprous (CuI), and cupric (CuII) oxides) was evaluated for the phiX174 bacteriophage under visible light illumination (Vis/Cu-TiO2). CuI-TiO2 exhibited superior virucidal activity (5 log inactivation in 30 min) and reusability (only 11 % loss of activity in the fifth cycle) compared to Cu0-TiO2 and CuII-TiO2. Photoluminescence spectroscopy and photocurrent measurements showed that CuI-TiO2 exhibited the highest charge separation efficiency and photocurrent density (approximately 0.24 μA/cm2) among the three materials, resulting in the most active redox reactions of Cu. Viral inactivation tests under different additives and viral particle integrity analyses (i.e., protein oxidation and DNA damage analyses) revealed that different virucidal species played key roles in the three Vis/Cu-TiO2 systems; Cu(III) was responsible for the viral inactivation by Vis/CuI-TiO2. The Vis/CuI-TiO2 system exhibited substantial virucidal performance for different viral species and in different water matrices, demonstrating its potential practical applications. The findings of this study offer valuable insights into the design of effective and sustainable antiviral photocatalysts for disinfection.