Publication
Advanced Redox Technology Lab
Publication
Advanced Redox Technology Lab
Journal papers
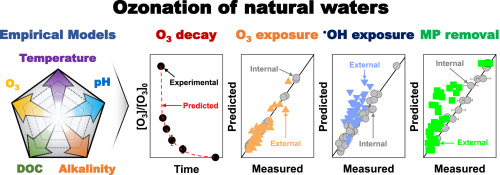
This study demonstrates new empirical models to predict the decomposition of ozone (O3) and the exposures of oxidants (i.e., O3 and hydroxyl radical, radical dotOH) during the ozonation of natural waters. Four models were developed for the instantaneous O3 demand, first-order rate constant for the secondary O3 decay, O3 exposure (∫[O3]dt), and radical dotOH exposure ((∫[radical dotOH]dt)), as functions of five independent variables, namely the O3 dose, concentration of dissolved organic carbon (DOC), pH, alkalinity, and temperature. The models were derived by polynomial regression analysis of experimental data obtained by controlling variables in natural water samples from a single source water (Maegok water in Korea), and they exhibited high accuracies for regression (R2 = 0.99 for the three O3 models, and R2 = 0.96 for the radical dotOH exposure model). The three O3 models exhibited excellent internal validity for Maegok water samples of different conditions (that were not used for the model development). They also showed acceptable external validity for seven natural water samples collected from different sources (not Maegok water); the IOD model showed somewhat poor external validity. However, the radical dotOH exposure model showed relatively poor internal and external validity. The models for oxidant exposures were successfully used to predict the abatement of micropollutants by ozonation; the model predictions showed high accuracy for Maegok water, but not for the other natural waters.