Publication
Advanced Redox Technology Lab
Publication
Advanced Redox Technology Lab
Journal papers
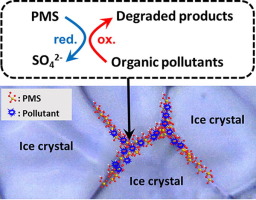
This study presents a freezing method for accelerating the peroxymonosulfate (PMS)-mediated degradation process. The degradation of furfuryl alcohol (FFA) in the presence of PMS was markedly accelerated by freezing. The degradation efficiency of FFA was only 10.4% in aqueous solution at 25 °C, but 100% degradation was achieved in frozen solution at −20 °C after 3 h of reaction at [FFA] = 20 µM and [PMS] = 100 µM. This accelerated PMS-mediated degradation of FFA in the frozen solution is due to the concentration of both PMS and FFA in ice grain boundaries, which increases the collision frequency between PMS and FFA thereby facilitating redox transformation. The mapping images of PMS and FFA in the frozen sample obtained using confocal Raman microscopy provide clear evidence of the accumulation of PMS and FFA in the ice grain boundaries after freezing. The experimental results with sulfate radical (SO4radical dot−) scavengers, no production of hydroxyl radicals (radical ·OH) and SO4 radical dot−, and the highly pollutant-dependent degradation efficiency suggest that the PMS-mediated degradation in frozen solution primarily proceeds through the direct electron transfer from organic pollutants to PMS (non-radical mechanism) rather than the reaction of SO4 radical dot− or radical dotOH with organic pollutants (radical mechanism). The degradation efficiency of the PMS/freezing system was similar across the pH range of 3–10. In addition, the PMS/freezing system worked efficiently in the temperature range of −10 to −35 °C. This result implies that the PMS/freezing system can be operated without external energy in cold regions.