Publication
Advanced Redox Technology Lab
Publication
Advanced Redox Technology Lab
Journal papers
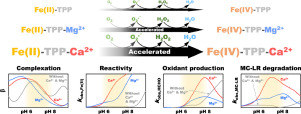
Fe(II)-tetrapolyphosphate complexes are known to activate molecular oxygen (Fe(II)-TPP/O2) to produce reactive oxidants (most likely, Fe(IV)-TPP complexes) that are capable of degrading refractory organic contaminants in water. This study found that magnesium and calcium ions (Mg2+ and Ca2+) accelerate the degradation of micfrocystin-LR (MC-LR), the most toxic and abundant cyanotoxin, by the Fe(II)-TPP/O2 system. The addition of Mg2+ and Ca2+ increased the observed rate constant of MC-LR degradation by up to 4.3 and 14.8 folds, respectively. Mg2+ and Ca2+ accelerated the MC-LR degradation in the entire pH range, except for the alkaline region with pH > ca. 10. The addition of Mg2+ and Ca2+ also reshaped the pH-dependency of the MC-LR degradation, greatly increasing the rate of MC-LR degradation at neutral pH. It was found that Mg2+ and Ca2+ accelerate the reaction of Fe(II)-TPP complexes with oxygen, resulting in faster production of reactive oxidants. The findings from cyclic voltammetry and potentiometric titration suggest that Mg2+ and Ca2+ form ternary complexes with Fe(II)-TPP, which exhibit higher reactivity with oxygen. Due to the effects of Mg2+ and Ca2+, the rate of MC-LR degradation by the Fe(II)-TPP/O2 system was even higher in natural water than in deionized water.