Publication
Advanced Redox Technology Lab
Publication
Advanced Redox Technology Lab
Journal papers
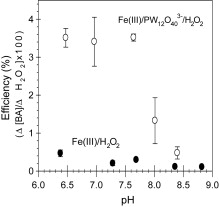
Ferric ion (Fe[III]) catalyzes the decomposition of hydrogen peroxide (H2O2) into strong oxidants such as hydroxyl radical ( OH) and ferryl ion (Fe[IV]) through the redox cycling of the iron couple (Fe[II]/Fe[III]). The use of these reactions for the catalytic oxidation of organic compounds is usually limited to the acidic pH region due to the low solubility of Fe(III) and the low efficiency of oxidant production at neutral pH values. The addition of phosphotungstate (PW12O403−), a polyoxometalate, extends the working pH range of the Fe(III)/H2O2 system up to pH 8.5. PW12O403− forms a soluble complex with iron that converts H2O2 into oxidants. The coordination of Fe(II) by PW12O403− also alters the mechanism of the reaction of Fe(II) with H2O2 at neutral pH, resulting in formation of an oxidant capable of oxidizing aromatic compounds. The base-catalyzed hydrolysis of PW12O403− gradually results in inactivation of the catalyst. In the absence of Fe(III), PW12O403− was completely hydrolyzed after 1 day at pH 7.5, whereas the Fe(III)–PW12O403− complex was active for at least 4 days under the same conditions.
OH) and ferryl ion (Fe[IV]) through the redox cycling of the iron couple (Fe[II]/Fe[III]). The use of these reactions for the catalytic oxidation of organic compounds is usually limited to the acidic pH region due to the low solubility of Fe(III) and the low efficiency of oxidant production at neutral pH values. The addition of phosphotungstate (PW12O403−), a polyoxometalate, extends the working pH range of the Fe(III)/H2O2 system up to pH 8.5. PW12O403− forms a soluble complex with iron that converts H2O2 into oxidants. The coordination of Fe(II) by PW12O403− also alters the mechanism of the reaction of Fe(II) with H2O2 at neutral pH, resulting in formation of an oxidant capable of oxidizing aromatic compounds. The base-catalyzed hydrolysis of PW12O403− gradually results in inactivation of the catalyst. In the absence of Fe(III), PW12O403− was completely hydrolyzed after 1 day at pH 7.5, whereas the Fe(III)–PW12O403− complex was active for at least 4 days under the same conditions.