Publication
Advanced Redox Technology Lab
Publication
Advanced Redox Technology Lab
Journal papers
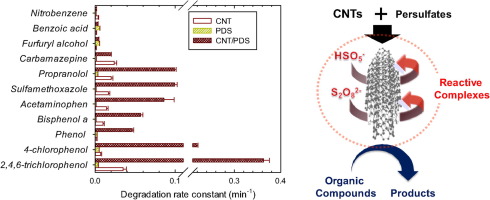
Carbon nanotubes (CNTs) have been found to activate persulfates (i.e., peroxymonosulfate and peroxydisulfate) into reactive species that are capable of oxidizing organic compounds in water. In the presence of single- or multi-walled CNTs, persulfates effectively degraded phenolic compounds and certain pharmaceuticals. Phenyl derivatives substituted with electron-withdrawing groups, such as benzoic acid and nitrobenzene, were resistant to degradation by the CNT/persulfate system. Based on observations regarding persulfate decomposition and linear sweep voltammetry using a CNT electrode, it has been suggested that persulfates bind onto the surface of CNTs, forming reactive complexes that are immediately decomposed upon reaction with organic compounds. Electron paramagnetic resonance spectroscopy with spin-trapping indicates that these reactive species are distinct from sulfate radical anions or hydroxyl radicals. The CNT-activated persulfate system shows promise as a novel treatment technology for the selective oxidation of organic contaminants in water.