Publication
Advanced Redox Technology Lab
Publication
Advanced Redox Technology Lab
Journal papers
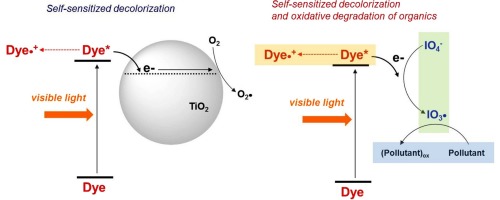
Inspired by the mechanism behind self-sensitized destruction of dyes on semiconductor photocatalysts, we herein present the first instance of visible-light-induced activation of periodate (IO4−) into reactive iodine radicals via sensitized electron transfer from an organic dye, Rhodamine B (RhB). The IO4− reduction not only leads to oxidative decolorization of RhB but also formation of reactive intermediates that degrade organic compounds. Electron transfer from the excited dye to IO4− was confirmed by detecting RhB radical cation (RhBradical dot+) and measuring its lifetime. The efficiency of organic compound degradation was found to significantly vary depending on the target substrate, i.e., phenol, bisphenol A, and 4-chlorophenol were rapidly decomposed, whereas benzoic acid, carbamazepine, 4-nitrophenol, and sulfamethoxazole exhibited moderate decomposition rate. Lines of evidence in addition to the substrate specificity, such as insignificant hydroxylation, non-stoichiometric dechlorination, and marginal quenching effects of organic/inorganic compounds (e.g., methanol, natural organic matters, and chloride ion), points toward the involvement of iodate radical (IO3radical dot). The dye-sensitized IO4− activation process was also found to be highly effective in inactivation of MS2 bacteriophage.