Publication
Advanced Redox Technology Lab
Publication
Advanced Redox Technology Lab
Journal papers
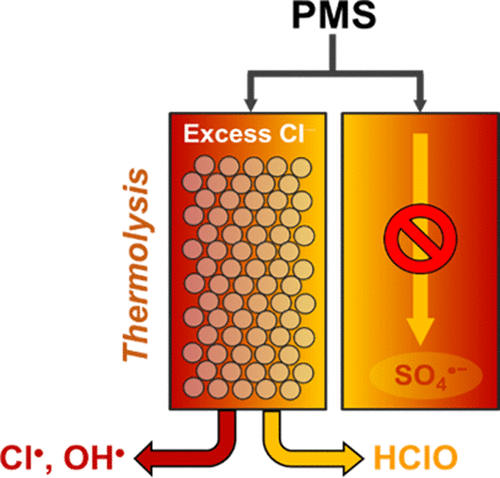
This study is the first to demonstrate the capability of Cl– to markedly accelerate organic oxidation using thermally activated peroxymonosulfate (PMS) under acidic conditions. The treatment efficiency gain allowed heat-activated PMS to surpass heat-activated peroxydisulfate (PDS). During thermal PMS activation at excess Cl–, accelerated oxidation of 4-chlorophenol (susceptible to oxidation by hypochlorous acid (HOCl)) was observed along with significant degradation of benzoic acid and ClO3– occurrence, which involved oxidants with low substrate specificity. This indicated that heat facilitated HOCl formation via nucleophilic Cl– addition to PMS and enabled free chlorine conversion into less selective oxidizing radicals. HOCl acted as a key intermediate in the major oxidant transition based on temperature-dependent variation in HOCl concentration profiles, kinetically retarded organic oxidation upon NH4+ addition, and enabled rapid organic oxidation in heated PMS/HOCl mixtures. Chlorine atom that formed via the one-electron oxidation of Cl– by the sulfate radical served as the primary oxidant and was involved in hydroxyl radical production. This was corroborated by the quenching effects of alcohols and bicarbonates, reactivity toward multiple organics, and electron paramagnetic resonance spectral features. PMS outperformed PDS in degrading benzoic acid during thermal activation operated in reverse osmosis concentrate, which was in conflict with the well-established superiority of heat-activated PDS.