Publication
Advanced Redox Technology Lab
Publication
Advanced Redox Technology Lab
Journal papers
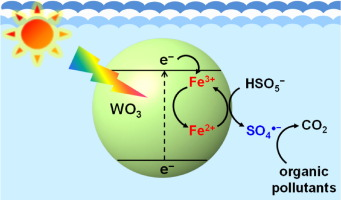
The effect of ferric ions (Fe3+) on the degradation of organic pollutants through the photocatalytic activation of peroxymonosulfate (PMS) was investigated. In the presence of Fe3+, the degradation of 4-chlorophenol (4-CP) was significantly enhanced in the PMS/tungsten oxide (WO3) system under visible light irradiation. The enhanced degradation efficiency by Fe3+ is primarily ascribed to the role of Fe3+ as an electron-transfer mediator, as it induces a cascadal electron transfer from WO3 to Fe3+ to PMS. The production of the sulfate radical (SO4radical dot−) and its significant contribution to the degradation of 4-CP in the Fe3+/PMS/WO3 system were verified via electron paramagnetic resonance (EPR) spectroscopy and degradation experiments using radical scavengers (methanol and tert-butyl alcohol), respectively. The addition of Fe3+ to the PMS/WO3 system also significantly increased the degradation rate of other organic pollutants, such as phenol, 2,4-dimethylphenol, bisphenol A, sulfamethoxazole, sulfanilamide, and propranolol. The degradation efficiency of the Fe3+/PMS/WO3 system was largely maintained in multiple degradation cycles by the regular addition of PMS only. In addition, the Fe3+/PMS/WO3 system exhibited a higher degradation efficiency than the conventional cobaltous ion (Co2+)/PMS system at equal transition metal ion concentrations. The addition of Fe3+ for the purpose of enhancing the degradation efficiency is not restricted to the PMS activation system. This method can be applied to the activation of other oxyanions, such as peroxydisulfate (S2O82−) and bromate (BrO3−), for wastewater treatment.