Publication
Advanced Redox Technology Lab
Publication
Advanced Redox Technology Lab
Journal papers
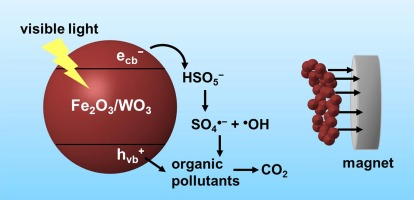
Developing a cost-effective method for the separation and recovery of nanomaterials for water treatment is challenging due to the significantly high recovery cost associated with nanomaterial-based water treatment processes. In this study, we synthesized magnetically separable iron oxide/tungsten oxide (Fe2O3/WO3) composites through a chemical co-precipitation method and employed them as peroxymonosulfate (PMS) activators for the degradation of organic pollutants under visible light. Electron spin resonance analysis, chemical quenching, and photoluminescence analysis suggested that hydroxyl radicals, sulfate radicals, and valence band holes contribute to the degradation processes in the Fe2O3/WO3/PMS system under visible light. The results of the degradation and recovery experiments based on the mass ratio of WO3 to Fe2O3 ([WO3]:[Fe2O3]) in the Fe2O3/WO3 composite indicated that the Fe2O3/WO3 composite with [WO3]:[Fe2O3] = 2:1, which can be completely recovered using a magnet (magnetic flux density = 2500 G), is the optimal photocatalyst for PMS activation. The effects of reaction parameters, such as the PMS concentration, Fe2O3/WO3 dosage, solution pH, and background anions (chloride, carbonate, nitrate, and phosphate)/humic acid, on the degradation kinetics were investigated and discussed. Under visible light irradiation, the Fe2O3/WO3/PMS system efficiently degraded various organic pollutants, including 4-chlorophenol, 2-chlorophenol, 4-bromophenol, 4-methylphenol, 2,4-dimethylphenol, bisphenol A, tryptophan, sulfamethoxazole, sulfisoxazole, and furfuryl alcohol. In addition, the complete degradation of 4-chlorophenol in the Fe2O3/WO3/PMS system under visible light was achieved for up to five degradation cycles when Fe2O3/WO3 was reused after recovery using a magnet.