Short Review of Multichannel Membrane Capacitive Deionization: Principle, Current Status, and Future Prospect
Abstract
:1. Introduction
2. Capacitive Deionization (CDI)
2.1. Basic Principle and Operational Features
2.2. Standard Analysis Metrics
2.3. Operational Mode
2.4. Advancement of Capacitive Deionization (CDI) and Its Limitations
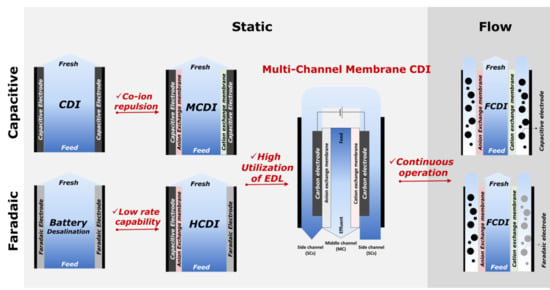
3. Multi-Channel Membrane CDI (MC-MCDI)
3.1. Cell Configuration and Key Advantages
3.2. Operational Studies and Principle of Mechanism
3.3. Applications of MC-MCDI
4. Conclusions and Future Prospect
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Suss, M.; Porada, S.; Sun, X.; Biesheuvel, P.; Yoon, J.; Presser, V. Water desalination via capacitive deionization: What is it and what can we expect from it? Energy Environ. Sci. 2015, 8, 2296–2319. [Google Scholar] [CrossRef] [Green Version]
- Porada, S.; Zhao, R.; Van Der Wal, A.; Presser, V.; Biesheuvel, P. Review on the science and technology of water desalination by capacitive deionization. Prog. Mater. Sci. 2013, 58, 1388–1442. [Google Scholar] [CrossRef] [Green Version]
- AlMarzooqi, F.A.; Al Ghaferi, A.A.; Saadat, I.; Hilal, N. Application of capacitive deionisation in water desalination: A review. Desalination 2014, 342, 3–15. [Google Scholar] [CrossRef]
- Yoon, H.; Lee, J.; Kim, S.; Yoon, J. Review of concepts and applications of electrochemical ion separation (EIONS) process. Sep. Purif. Technol. 2018, 215, 190–207. [Google Scholar] [CrossRef]
- Anderson, M.A.; Cudero, A.L.; Palma, J. Capacitive deionization as an electrochemical means of saving energy and delivering clean water. Comparison to present desalination practices: Will it compete? Electrochim. Acta 2010, 55, 3845–3856. [Google Scholar] [CrossRef]
- Zhao, R.; Porada, S.; Biesheuvel, P.; Van der Wal, A. Energy consumption in membrane capacitive deionization for different water recoveries and flow rates, and comparison with reverse osmosis. Desalination 2013, 330, 35–41. [Google Scholar] [CrossRef]
- Zhang, D.; Yan, T.; Shi, L.; Peng, Z.; Wen, X.; Zhang, J. Enhanced capacitive deionization performance of graphene/carbon nanotube composites. J. Mater. Chem. 2012, 22, 14696–14704. [Google Scholar] [CrossRef]
- Kim, C.; Lee, J.; Kim, S.; Yoon, J. TiO2 sol–gel spray method for carbon electrode fabrication to enhance desalination efficiency of capacitive deionization. Desalination 2014, 342, 70–74. [Google Scholar] [CrossRef]
- Lee, L.Y.; Ng, H.Y.; Ong, S.L.; Tao, G.; Kekre, K.; Viswanath, B.; Lay, W.; Seah, H. Integrated pretreatment with capacitive deionization for reverse osmosis reject recovery from water reclamation plant. Water Res. 2009, 43, 4769–4777. [Google Scholar] [CrossRef]
- Tan, C.; He, C.; Tang, W.; Kovalsky, P.; Fletcher, J.; Waite, T.D. Integration of photovoltaic energy supply with membrane capacitive deionization (MCDI) for salt removal from brackish waters. Water Res. 2018, 147, 276–286. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, C.; Liu, X.; Xu, X.; Sun, Z.; Pan, L. Review on carbon-based composite materials for capacitive deionization. RSC Adv. 2015, 5, 15205–15225. [Google Scholar] [CrossRef]
- Jia, B.; Zhang, W. Preparation and application of electrodes in capacitive deionization (CDI): A state-of-art review. Nanoscale Res. Lett. 2016, 11, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Zhang, Y.; Bao, S.; Song, S. Desalination by capacitive deionization with carbon-based materials as electrode: A review. Surf. Rev. Lett. 2013, 20, 1330003. [Google Scholar] [CrossRef]
- Oladunni, J.; Zain, J.H.; Hai, A.; Banat, F.; Bharath, G.; Alhseinat, E. A comprehensive review on recently developed carbon based nanocomposites for capacitive deionization: From theory to practice. Sep. Purif. Technol. 2018, 207, 291–320. [Google Scholar] [CrossRef]
- Kim, C.; Lee, J.; Srimuk, P.; Aslan, M.; Presser, V. Concentration-gradient multichannel flow-stream membrane capacitive deionization cell for high desalination capacity of carbon electrodes. ChemSusChem 2017, 10, 4914–4920. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S.; Kim, C.; Yoon, J. Hybrid capacitive deionization to enhance the desalination performance of capacitive techniques. Energy Environ. Sci. 2014, 7, 3683–3689. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Yoon, J. Rocking chair desalination battery based on prussian blue electrodes. ACS Omega 2017, 2, 1653–1659. [Google Scholar] [CrossRef]
- Kim, C.; Srimuk, P.; Lee, J.; Fleischmann, S.; Aslan, M.; Presser, V. Influence of pore structure and cell voltage of activated carbon cloth as a versatile electrode material for capacitive deionization. Carbon 2017, 122, 329–335. [Google Scholar] [CrossRef]
- Srimuk, P.; Kaasik, F.; Krüner, B.; Tolosa, A.; Fleischmann, S.; Jäckel, N.; Tekeli, M.C.; Aslan, M.; Suss, M.E.; Presser, V. MXene as a novel intercalation-type pseudocapacitive cathode and anode for capacitive deionization. J. Mater. Chem. A 2016, 4, 18265–18271. [Google Scholar] [CrossRef] [Green Version]
- Srimuk, P.; Husmann, S.; Presser, V. Low voltage operation of a silver/silver chloride battery with high desalination capacity in seawater. RSC Adv. 2019, 9, 14849–14858. [Google Scholar] [CrossRef] [Green Version]
- Farmer, J.C.; Fix, D.V.; Mack, G.V.; Pekala, R.W.; Poco, J.F. Capacitive deionization of NaCl and NaNO3 solutions with carbon aerogel electrodes. J. Electrochem. Soc. 1996, 143, 159–169. [Google Scholar] [CrossRef]
- Suss, M.E.; Baumann, T.F.; Bourcier, W.L.; Spadaccini, C.M.; Rose, K.A.; Santiago, J.G.; Stadermann, M. Capacitive desalination with flow-through electrodes. Energy Environ. Sci. 2012, 5, 9511–9519. [Google Scholar] [CrossRef]
- Gao, X.; Omosebi, A.; Landon, J.; Liu, K. Surface charge enhanced carbon electrodes for stable and efficient capacitive deionization using inverted adsorption–desorption behavior. Energy Environ. Sci. 2015, 8, 897–909. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Kim, C.; Yoon, J. Na2FeP2O7 as a novel material for hybrid capacitive deionization. Electrochim. Acta 2016, 203, 265–271. [Google Scholar] [CrossRef]
- Smith, K.C.; Dmello, R. Na-ion desalination (NID) enabled by Na-blocking membranes and symmetric Na-intercalation: Porous-electrode modeling. J. Electrochem. Soc. 2016, 163, A530–A539. [Google Scholar] [CrossRef]
- Pasta, M.; Wessells, C.D.; Cui, Y.; La Mantia, F. A desalination battery. Nano Lett. 2012, 12, 839–843. [Google Scholar] [CrossRef]
- Jeon, S.-I.; Park, H.-R.; Yeo, J.-G.; Yang, S.; Cho, C.H.; Han, M.H.; Kim, D.K. Desalination via a new membrane capacitive deionization process utilizing flow-electrodes. Energy Environ. Sci. 2013, 6, 1471–1475. [Google Scholar] [CrossRef]
- Doornbusch, G.; Dykstra, J.; Biesheuvel, P.; Suss, M. Fluidized bed electrodes with high carbon loading for water desalination by capacitive deionization. J. Mater. Chem. A 2016, 4, 3642–3647. [Google Scholar] [CrossRef]
- Tang, W.; He, D.; Zhang, C.; Kovalsky, P.; Waite, T.D. Comparison of Faradaic reactions in capacitive deionization (CDI) and membrane capacitive deionization (MCDI) water treatment processes. Water Res. 2017, 120, 229–237. [Google Scholar] [CrossRef]
- Biesheuvel, P.; Zhao, R.; Porada, S.; Van der Wal, A. Theory of membrane capacitive deionization including the effect of the electrode pore space. J. Colloid Interface Sci. 2011, 360, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Porada, S.; Borchardt, L.; Oschatz, M.; Bryjak, M.; Atchison, J.; Keesman, K.; Kaskel, S.; Biesheuvel, P.; Presser, V. Direct prediction of the desalination performance of porous carbon electrodes for capacitive deionization. Energy Environ. Sci. 2013, 6, 3700–3712. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Srimuk, P.; Lee, J.; Aslan, M.; Presser, V. Semi-continuous capacitive deionization using multi-channel flow stream and ion exchange membranes. Desalination 2018, 425, 104–110. [Google Scholar] [CrossRef]
- Kim, C.; Srimuk, P.; Lee, J.; Presser, V. Enhanced desalination via cell voltage extension of membrane capacitive deionization using an aqueous/organic bi-electrolyte. Desalination 2018, 443, 56–61. [Google Scholar] [CrossRef]
- Kim, N.; Hong, S.P.; Lee, J.; Kim, C.; Yoon, J. High-desalination performance via redox couple reaction in the multichannel capacitive deionization system. ACS Sustain. Chem. Eng. 2019, 7, 16182–16189. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.; Ahn, J.; Jo, K.; Hong, S.P.; Kim, C.; Lee, C.; Yoon, J. Enhancement in desalination performance of battery electrodes via improved mass transport using a multichannel flow system. ACS Appl. Mater. Interfaces 2019, 11, 36580–36588. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kim, T.; Shin, H.; Lee, J.; Ha, J.-I.; Yoon, J. Direct energy recovery system for membrane capacitive deionization. Desalination 2016, 398, 144–150. [Google Scholar] [CrossRef]
- Tan, C.; He, C.; Fletcher, J.; Waite, T.D. Energy recovery in pilot scale membrane CDI treatment of brackish waters. Water Res. 2019, 168, 115146. [Google Scholar] [CrossRef]
- Zhao, R.; Satpradit, O.; Rijnaarts, H.; Biesheuvel, P.; Van der Wal, A. Optimization of salt adsorption rate in membrane capacitive deionization. Water Res. 2013, 47, 1941–1952. [Google Scholar] [CrossRef]
- Kim, T.; Yoon, J. CDI ragone plot as a functional tool to evaluate desalination performance in capacitive deionization. RSC Adv. 2015, 5, 1456–1461. [Google Scholar] [CrossRef]
- Zhao, R.; Biesheuvel, P.; Van der Wal, A. Energy consumption and constant current operation in membrane capacitive deionization. Energy Environ. Sci. 2012, 5, 9520–9527. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Dykstra, J.; Lin, S. Energy efficiency of capacitive deionization. Environ. Sci. Technol. 2019, 53, 3366–3378. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Lee, J.; Kim, S.; Kim, C.; Lee, J.; Biesheuvel, P.; Yoon, J. High performance electrochemical saline water desalination using silver and silver-chloride electrodes. Desalination 2020, 476, 114216. [Google Scholar] [CrossRef]
- Tang, W.; Liang, J.; He, D.; Gong, J.; Tang, L.; Liu, Z.; Wang, D.; Zeng, G. Various cell architectures of capacitive deionization: Recent advances and future trends. Water Res. 2019, 150, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, C.; Lee, J.; Kim, S.; Lee, J.; Kim, J.; Yoon, J. Hybrid electrochemical desalination system combined with an oxidation process. ACS Sustain. Chem. Eng. 2018, 6, 1620–1626. [Google Scholar] [CrossRef]
- Porada, S.; Sales, B.; Hamelers, H.; Biesheuvel, P. Water desalination with wires. J. Phys. Chem. Lett. 2012, 3, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wang, G.; Zhan, F.; Dong, Q.; Ren, Q.; Wang, J.; Qiu, J. Surface-treated carbon electrodes with modified potential of zero charge for capacitive deionization. Water Res. 2016, 93, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Aslan, M.; Zeiger, M.; Jäckel, N.; Grobelsek, I.; Weingarth, D.; Presser, V. Improved capacitive deionization performance of mixed hydrophobic/hydrophilic activated carbon electrodes. J. Phys. Condens. Matter 2016, 28, 114003. [Google Scholar] [CrossRef]
- Gao, X.; Porada, S.; Omosebi, A.; Liu, K.-L.; Biesheuvel, P.; Landon, J. Complementary surface charge for enhanced capacitive deionization. Water Res. 2016, 92, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wang, D.; Ji, L.; Gong, Q.; Zhu, Y.; Liang, J. Equilibrium and kinetic studies on the removal of NaCl from aqueous solutions by electrosorption on carbon nanotube electrodes. Sep. Purif. Technol. 2007, 58, 12–16. [Google Scholar] [CrossRef]
- Xu, X.; Sun, Z.; Chua, D.H.; Pan, L. Novel nitrogen doped graphene sponge with ultrahigh capacitive deionization performance. Sci. Rep. 2015, 5, 11225. [Google Scholar] [CrossRef]
- Lee, J.; Srimuk, P.; Carpier, S.; Choi, J.; Zornitta, R.L.; Kim, C.; Aslan, M.; Presser, V. Confined redox reactions of iodide in carbon nanopores for fast and energy-efficient desalination of brackish water and seawater. ChemSusChem 2018, 11, 3460–3472. [Google Scholar] [CrossRef] [PubMed]
- Akinwolemiwa, B.; Peng, C.; Chen, G.Z. Redox electrolytes in supercapacitors. J. Electrochem. Soc. 2015, 162, A5054–A5059. [Google Scholar] [CrossRef]









| Unit | Definition | |
|---|---|---|
| Salt adsorption capacity (SAC) | mg/gelectrode | Ion removal capacity |
| Salt removal rate (SAR) | mg/gelectrode/s | Rate capability |
| Charge efficiency | % | Ratio of removed ions to the invested electric charge |
| Energy consumption | kT | Energy consumption per removed ion |
| System Operation Mode | Current | Voltage |
|---|---|---|
| Charging | Constant current (CC) | Constant voltage (CV) |
| Discharging | Reversed current | Zero voltage |
| Reversed voltage |
| Mode | Material | Cell Voltage (V) | Feed Concentration (mM) | SAC (mg/g)/Energy Consumption (kT) | Charge Efficiency | Ref. |
|---|---|---|---|---|---|---|
| MCDI | AC | +1.2/0 | 20 | 11.7/n.a. | [38] | |
| +1.2/−1.2 | 20 | 13.4/n.a. | [30] | |||
| +1.2/0 | 100 | 6.5/24 | ||||
| +1.2/−1.2 | 8.5 | 19.5/n.a. | [46] | |||
| +1.2/0 | 5 | 10.3/n.a. | [47] | |||
| CDI | ACC | +1.2/0 | 5 | 16.2/n.a. | [18] | |
| Chemical treated ACC | +1.0/0 | 5 | 15.4/n.a. | [48] | ||
| Carbon nanotubes | +1.2/0 | 8.5 | 2.5/n.a. | |||
| Carbon nanotubes | +1.2/0 | 34 | 5/n.a. | [49] | ||
| Graphene sponge | +1.2/0 | 8.5 | 14.6/n.a. | [50] | ||
| MC-MCDI | ACC(Kynol) | +1.2/0 | M: 5 (S: 5) | 14.1/~20 | >90 | [15] |
| +1.2/−1.2 | 22.6/~20 | |||||
| +1.2/0 | M: 5 (S: 1000) | 29.8/~20 | ||||
| +1.2/−1.2 | 56.8/~20 | |||||
| ACC(Kuraray) | +1.2/0 | M: 10 (S: 100 NaCl, 100Na4Fe(CN)6) | 67.8/~27 | >90 | [34] | |
| Prussian blue | +1.0/−1.0 | M: 10 (S: 1000) | 52.9/~38 | ~98 | [35] | |
| ACC(Kynol) | +2.4/0.0 | M: 5 (S: 1 M NaCl in H2O, 1 M NaClO4 in PC) | 63.5/27 | ~90 | [33] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.; Lee, J.; Kim, S.; Hong, S.P.; Lee, C.; Yoon, J.; Kim, C. Short Review of Multichannel Membrane Capacitive Deionization: Principle, Current Status, and Future Prospect. Appl. Sci. 2020, 10, 683. https://doi.org/10.3390/app10020683
Kim N, Lee J, Kim S, Hong SP, Lee C, Yoon J, Kim C. Short Review of Multichannel Membrane Capacitive Deionization: Principle, Current Status, and Future Prospect. Applied Sciences. 2020; 10(2):683. https://doi.org/10.3390/app10020683
Chicago/Turabian StyleKim, Nayeong, Jiho Lee, Seonghwan Kim, Sung Pil Hong, Changha Lee, Jeyong Yoon, and Choonsoo Kim. 2020. "Short Review of Multichannel Membrane Capacitive Deionization: Principle, Current Status, and Future Prospect" Applied Sciences 10, no. 2: 683. https://doi.org/10.3390/app10020683