Publication
Advanced Redox Technology Lab
Publication
Advanced Redox Technology Lab
Journal papers
Electrochemical oxidation processes (EOPs) have
gained attention as promising technologies for the removal of
refractory organic pollutants in water and wastewater. Although
many reviews have been reported on EOPs, to date, no review has
been mainly focused on the energy efficiencies of EOPs.
Accordingly, herein, EOPs for the treatment of organic pollutants
in water and wastewater are reviewed, and the performances of
different EOPs in terms of energy efficiencies are evaluated using
various figures of merit. The basic principles of EOPs are briefly
introduced, and indicators for the performance evaluation of EOPs
are analyzed based on the fundamentals and statistical databases of
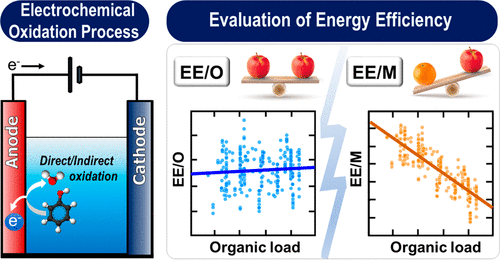
EOPs obtained from the literature. Electrical energy per order
(EE/O) and electrical energy per mass (EE/M) have been widely
used to measure the energy efficiencies of EOPs. However, statistical analysis indicates that EE/O is a more suitable index in this
regard than EE/M because it better represents the kinetics of pollutant degradation by EOPs, thereby mitigating the influence of the
initial organic load on the performance evaluation of EOPs. EOPs modified using Fenton’s reagent or external supplies of energy (or
chemicals) exhibit higher energy efficiencies than those of anodic oxidation processes due to the existence of additional reactions or
synergistic mechanisms for the generation of reactive oxidants. In addition to the evaluation of the energy efficiencies of different
types of EOPs, the effects of electrode materials and electrolysis conditions, such as current density, pH, anions, and additive
concentrations, on the energy efficiencies of EOPs are discussed. We believe that our review will provide useful information and
insights for choosing the optimal types and conditions of EOPs for pollutant removal.