Publication
Advanced Redox Technology Lab
Publication
Advanced Redox Technology Lab
Journal papers
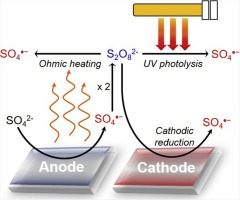
This study scrutinized the roles of sulfate radicals (SO4 radical dot−) and peroxydisulfate (PDS) formed from SO42− in electrochemical organic oxidation on a boron-doped diamond (BDD) electrode. The substrate-specific performance of electrochemical oxidation using SO42− as the electrolyte aligned with the reactivity of SO4radical dot− produced via radiolysis- or heat-induced PDS activation, but was distinct from the non-selective oxidation efficiency observed in an aqueous ClO4− solution. A comparison of the treatment efficiencies using different electrolytes (i.e., Cl−, SO42−, and ClO4−) showed no pronounced enhancing effect of SO4 radical dot− on the anodic oxidation of diverse organics (except perfluorooctanoate), which implied that direct electron transfer and hydroxyl radical-induced oxidation proceeded as complementary reaction routes. Repeated electrolytic oxidation caused substantial electrolyte exchange from Cl− to ClO4−, which retarded organic oxidation accompanied by ClO4− accumulation. Conversely, high-yield PDS production observed when SO42− was used instead barely reduced treatment efficiency. Together with SO4r adical dot− detection in the electron paramagnetic resonance spectrum, a correlation between 4-chlorophenol oxidation rate and the faradaic efficiency for SO42− formation, monitored in PDS solutions while varying the cathode material, suggested cathodic PDS activation. The electrocatalytic performance was demonstrated to be further improved with anodically formed PDS activation through naturally occurring resistive heating or combination with UV photolysis as a post-treatment step.