Publication
Advanced Redox Technology Lab
Publication
Advanced Redox Technology Lab
Journal papers
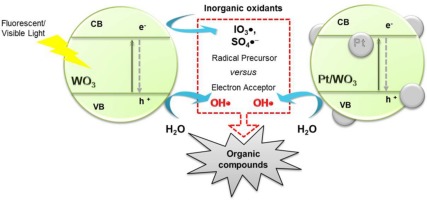
This study evaluates the capacity of various inorganic oxidants (IO4−, HSO5−, S2O82−, H2O2, and BrO3−) to act as alternative electron acceptors for WO3-mediated photocatalytic oxidation. Combination with IO4− drastically increased the rate of photocatalytic degradation of 4-chlorophenol by WO3, while the other oxyanions only negligibly improved the photocatalytic activity. The extent of the photocatalytic performance enhancement in the presence of inorganic oxidants correlated well with the efficiencies for: (1) hydroxylation of benzoic acid as an OH probe, (2) dechlorination of dichloroaceate as a hole scavenger, and (3) water oxidation with O2 evolution. The results suggest that the promoted charge separation primarily causes kinetic enhancement in photocatalytic degradation using the WO3/IO4− system. In marked contrast to the substrate-dependent activity of the photochemically activated IO4− (generating selective IO3
probe, (2) dechlorination of dichloroaceate as a hole scavenger, and (3) water oxidation with O2 evolution. The results suggest that the promoted charge separation primarily causes kinetic enhancement in photocatalytic degradation using the WO3/IO4− system. In marked contrast to the substrate-dependent activity of the photochemically activated IO4− (generating selective IO3 ), the efficiency of the WO3/IO4− system for photocatalytic degradation did not sensitively depend on the type of target organic compound, which implies the existence of a minor contribution of the photocatalytic reduction pathway associated with the production of IO3
), the efficiency of the WO3/IO4− system for photocatalytic degradation did not sensitively depend on the type of target organic compound, which implies the existence of a minor contribution of the photocatalytic reduction pathway associated with the production of IO3 as a secondary oxidant. On the other hand, the insignificant inhibitory effect of methanol as an OH
as a secondary oxidant. On the other hand, the insignificant inhibitory effect of methanol as an OH quencher may reveal the possible involvement of SO4
quencher may reveal the possible involvement of SO4 − in the improved photocatalytic activity of the WO3/HSO5− system. The alternative use of platinized WO3, where the interfacial electron transfer occurs in a concerted step, achieved a highly accelerated photocatalytic oxidation in the presence of HSO5− and polyoxometalates as electron scavengers. In particular, the surface loading of nanoscale platinum appeared to retard the reaction route for SO4
− in the improved photocatalytic activity of the WO3/HSO5− system. The alternative use of platinized WO3, where the interfacial electron transfer occurs in a concerted step, achieved a highly accelerated photocatalytic oxidation in the presence of HSO5− and polyoxometalates as electron scavengers. In particular, the surface loading of nanoscale platinum appeared to retard the reaction route for SO4 − generation associated with a one-electron transfer.
− generation associated with a one-electron transfer.