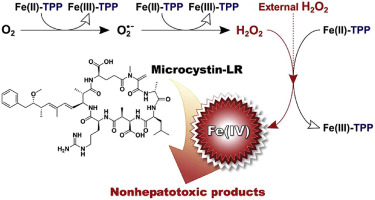
Ferrous-tetrapolyphosphate complexes (Fe(II)-TPP) activate oxygen and hydrogen peroxide to produce reactive oxidants capable of degrading organic compounds. In this study, the Fe(II)-TPP/O2 and Fe(II)-TPP/H2O2 systems were assessed for oxidative degradation of microcystin-LR (MC-LR), the most toxic and abundant cyanotoxin. The degradation of MC-LR was optimized for both the Fe(II)-TPP/O2 and Fe(II)-TPP/H2O2 systems when the molar ratio of TPP:Fe(II) was approximately 5.7–5.9. The optimal H2O2 dose for MC-LR degradation by Fe(II)-TPP/H2O2 was found to be 320 μM. The Fe(II)-TPP/O2 and Fe(II)-TPP/H2O2 systems exhibited two pH optima for MC-LR degradation i.e., ∼7 and 9, which can be attributed to pH-dependent reactivity changes of the resultant oxidants (most likely the ferryl-tetrapolyphostate complex, Fe(IV)-TPP). Liquid chromatography-mass spectrometry identified 22 compounds produced by the oxidation of MC-LR, including four primary oxidation products. One of the primary products, in particular, was formed via oxidative cleavage of the alkene group in the Mdha moiety of MC-LR. This compound and its secondary oxidation products are rarely found when MC-LR is transformed by other oxidants and is believed to reflect a unique reaction pathway involving Fe(IV)-TPP. Meanwhile, the hepatotoxicity of the reaction solution decreased concurrently with a decrease on MC-LR concentration.